Medical Devices Speaker Series 2023: How Cloud-Based In Silico Clinical Trials Accelerate Diabetes Monitoring Device Development
From the series: Medical Devices Speaker Series 2023
Alexandra Constantin, Tandem Diabetes Care
Developing innovative diabetes monitoring devices requires rigorous in silico testing and validation. But traditional clinical trials can be time-consuming and expensive. This presentation by Alex Constantin from Tandem Diabetes explores how cloud-based in silico clinical trials can revolutionize your development process.
Discover how MathWorks tools, combined with the power of the cloud, can enable you to:
- Create virtual patient populations: Develop diverse, realistic digital twins that accurately represent real-world patients.
- Conduct virtual clinical trials: Test your diabetes monitoring device in a simulated environment, gaining valuable insights into its performance and potential impact on patient outcomes.
- Optimize device design and functionality: Use the results of in silico trials to refine your device and ensure it meets the needs of patients and healthcare providers.
- Reduce development time and costs: Accelerate your path to market by complementing traditional clinical trials with efficient and cost-effective in silico trials.
This presentation was given at the 2023 MathWorks Medical Devices Speaker Series, which is a forum for researchers and industry practitioners using MATLAB® and Simulink® to showcase and discuss their state-of-the-art innovations in the areas of medical device research, prototyping, and compliance with FDA/MDR regulations for device certification.
About the presenter:
Dr. Alex Constantin is the senior director of data science and analytics at Tandem Diabetes Care, where she built a team of data scientists, data analysts, and data engineers focused on transforming data into value by developing a portfolio of data-centric products and services to reduce the burden of living with diabetes, improve customer experience, optimize business processes, and enable fast data-informed decisions. Dr. Constantin has dedicated her career to using data and algorithms to solve complex challenges in the healthcare industry. Before Tandem, she worked at Bigfoot Biomedical, Dexcom, and the National Institutes of Health. She has a Ph.D. in computer science from UC Berkeley, where she specialized in artificial intelligence and machine learning and focused on research in medical imagining.
Published: 18 May 2023
Hello, everyone. My name is Alex Constantin. It's a pleasure to be here speaking with you today about the use of simulations and medical device development in the diabetes space. Before I dive into that, I just wanted to clarify that today I'm speaking as an individual and not representing either my current employer, Tandem, or any of my past employers. But I'll give some examples from my experience working in the diabetes space.
So let's say that you already got a crash course in the risks of diabetes management from Laine. I will follow and tell you a little bit more about diabetes management. So I'm showing you some data here on the left from someone without diabetes, and on the right from someone with diabetes.
So diabetes is a condition where people don't get enough insulin, and their blood glucose rises. So in order to cover that rising blood glucose, insulin is provided exogenously. And the goal is to keep glucose in a very tight narrow glucose range.
So if you look at the images on the top, you see that gray band. That's a narrow glucose range that someone who is healthy is supposed to stay in. You see their glucose is fairly flat. That's at this range.
But on the right side with someone with diabetes, they often have excursions outside this range because it's really hard to balance what the body needs at all times. And there are many factors affecting this. If you look at the red range, that's low blood sugar. It can be really risky if it's left untreated. It can lead to confusion, coma, even death. So it's important to balance the risk of low blood sugar when administering insulin.
And the yellow range on top is high blood glucose. And chronic exposure to high blood glucose leads to microvascular complications, like kidney disease, trouble healing, and neuropathy, and nephropathy. So it's a balancing act. It's control engineering problem.
And if you look at the bottom graphs, you see the 50th to 75th percentile of blood glucose levels at the same time of day over a week. So you can see that someone who's healthy has almost a flat glucose profile with very, very little variability because their pancreas is really good at balancing the amount of insulin they need with the amount they actually receive.
But on the right side, someone with diabetes who's receiving treatment, they have much higher excursions, much higher variability. Like Laine said, the purpose is to transfer some of that variability burden to the user from that glycemic outcome to how the insulin gets delivered.
The reason this is hard, there are many, many factors affecting blood glucose levels. Some of them, we can measure, and some we can't. The biggest ones are carbohydrates and the insulin that gets delivered. And this is why today people with diabetes are expected to count carbohydrates and to use equations or calculators on their devices to figure out how much insulin to take to cover the carbohydrates that they're eating.
And this isn't easy. There's a lot of uncertainty. And people don't get it right all of the time, or maybe even a lot of the time, which is why some of the burden of diabetes comes from being constantly vigilant and thinking, well, did I make the right decision? Am I going low? Am I going high? Do I need to correct for it before it happens? How do I plan for my future activities, like exercise, and any of the other of those things that might affect blood glucose levels?
Novo Nordisk published this qualitative data of people with type 1 diabetes. They estimated that people make an average 180 extra health related decisions per day. That's a lot, which is why 6 in 10 struggled to turn off thinking about their diabetes, that need for control, that vigilance that keeps people needing to be constantly alert. And this is really hard to switch that off.
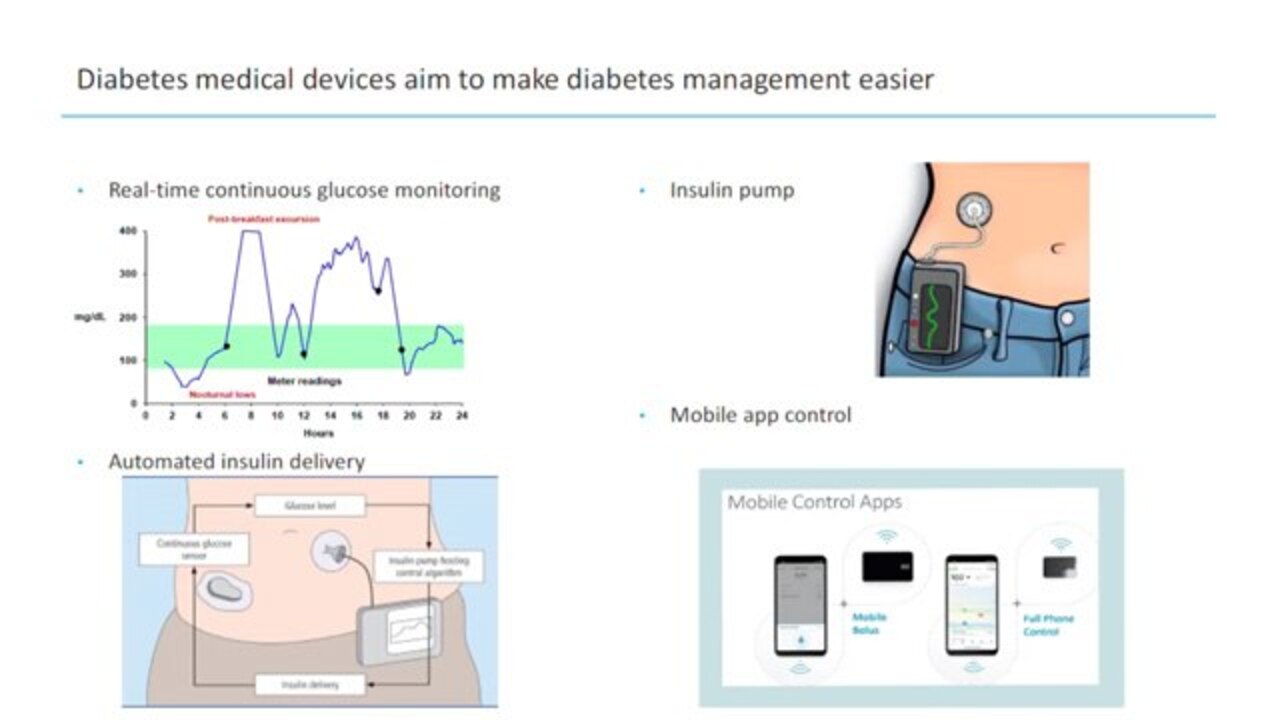
This is why over the past few decades a lot of progress has been made in medical devices that try to remove them. So we have continuous glucose monitors, like the one built by Dexcom or Abbott that provide almost constant readings of blood glucose levels throughout the day to make that monitoring easier and to make treatments be better informed, data informed.
Before this, people used to take 4 to 8 finger pricks a day to test their blood glucose. So you can see in this image above they could miss a lot of events of hypoglycemia, hyperglycemia, and really not do a very good job balancing the insulin they give with the insulin that the body needs. So CGM has provided this great benefit. They have alarms and alerts for safety. They can allow people to turn that vigilance off a little bit.
Insulin pumps allow people to take insulin through a continuous infusion without taking injections. This is also great. It allows a lot of flexibility in lifestyle that is not possible when you're on injections.
And recently, by integrating continuous glucose monitors with pumps and algorithms, it's possible to create automated insulin delivery systems that react to the glucose levels, and then just how much insulin someone receives in real time to keep their blood glucose in range and make it so that the human doesn't need to correct them and constantly look at it. So this has generated huge improvements in glycemic outcomes, and also a reduction in burden in the last few years that it's been on the market.
And now there's a lot of promise of improvements and usability from being able to control your smaller and smaller devices from a mobile app and leveraging everything that a mobile design has to offer to make that user experience easier.
So Laine talked a lot about all of the risks of diabetes management. So devices are taking on all of these burdens to do more for the user. How do we know that they're safe? Usually, the FDA has been looking at four types of evidence.
There's animal testing, which allows you to see gross biological responses. And it's usually one of the first things that was used for safety. There's bench testing, which allows you to examine the device under specific stress conditions, make sure that there are no mechanical issues with it, and that it performs the same under different conditions of load.
There are controlled clinical trials. They help you look at safety and effectiveness in very specific populations in a very controlled way. They're a great way to look at some of the physiological responses. But there's also more and more computational evidence is used. Simulations in clinical trials, which allow you to model specific situations, model specific risk cases, and compare things that you would otherwise not be able to do in clinical studies.
And the simulations complement existing methods to evaluate safety. This paper that shows how complementary they are is actually authored by someone from the FDA. And it's really helpful in seeing what clinical trials are good at versus what computer simulations are good at. Simulations allow you to predict performance in situations where it's not safe to sensitivity analysis, compare multiple devices on the same controls.
So this is why it's also with a condition that has 42 factors affecting variability. Simulations are a lot better than animal testing. And this is why over the last decade, the FDA has allowed the use of simulations before the first in human trial in lieu of animal testing, because they are really a lot better at evaluating risk than animal testing.
And the benefits of simulations, as I said, they're complementary. While a clinical trial gives you all of the possible things that may happen to a human that you may not have simulated, a simulation allows you to rapidly evaluate multiple algorithms, multiple possible designs to simulate a large population, look at at-risk populations without clinical trials recruitment bias. It allows you to isolate key variables and look at their full range, their full effect to simulate things beyond what's safe or what's expected to be seen in the clinical trials-- run large scale sensitivity analysis to set parameters.
So they're really useful at doing a lot of things that are not possible in clinical trials. But even as Laine has told you, they're also good at predicting and informing clinical trials, choosing the right sample sizes, the right primary outcomes to measure, and thinking, how long do I need to run this study for? What do I want to with the clinical study?
And so I'll give you some examples. So the first is an example from Dexcom. And Dexcom used simulations to get approval for a labeling change for their device. The Dexcom G5 continuous monitoring system at the beginning was approved. People could use it. They could get the safety of the alarms and the continuous information. But every time they needed to make a treatment decision, they had to confirm with a finger prick.
People started using the sensor to make treatment decisions, even though they weren't supposed to, according to the label. So the FDA knew this, and wanted to properly evaluate the safety of doing that. But it would have been really tough to do in a clinical study because it would be unethical to withhold CGM from people due to its benefits, and also people were already using it for treatment decisions. And they probably wouldn't have complied to testing with a finger prick every time.
So Dexcom went in front of an FDA panel and provided a lot of clinical evidence, testimonies from patients, but also a lot of simulations based on human physiology, models of accuracy for finger prick testing versus continuous glucose monitoring, and treatment, and behaviors to say, well, even though the point accuracy of finger pricks is higher, having continuous data, having alarms and alerts, being able to respond to all of that actually leads to better simulated outcomes than if you had four more accurate finger pricks per day. So this is one example where clinical trials would not have made this possible.
The other-- Laine told you from Bigfoot Biomedical predicting clinical trials. You'll see this again. But if you do your device development right, you can really do your simulations and know exactly what's going to happen in the trial.
So you see these outcomes. The clinical trial results are almost identical to the simulation results. And this is a sign that you've modeled all of the things of interest well, and that you can confidently make design decisions.
Another example, simulations can enables simpler and safer designs. And this is probably the area most excited about. Insulin pumps have more than 1,000 configurable parameters. Even AIB systems require all of these therapy settings things that are usually prescribed by physicians. And physicians spend about 70% of their time analyzing data and optimizing these settings.
It's a huge burden, and it's a barrier to adoption by some people and some care providers. So I'm not set up to do this, which is why my team at Tandem Diabetes Care developed an algorithm to automatically initialize and adapt these settings based on the data. We use simulations to do it. And our intention was to reduce the burden for diabetes educators to improve their time to holistic care, make the system more accessible to clinicians, primary care clinicians, who have less knowledge about settings, and don't want to assume as much responsibility over setting up and managing an insulin pump for their patients, and also for the people with diabetes on boarding with these devices to make their experience easier and optimize their glycemic outcomes as well as possible.
So we used simulations to develop this, and then ran a feasibility clinical trial that confirmed the results of the simulation. This was presented at ATTD recently by the clinical trial investigator, Dr. Shah. And it basically showed an improvement of 23% in 10 min range from the time people on multiple daily injections started on the pump to the end of the study, and also a 1% reduction in hypoglycemia.
And what's really great for this population for which who were using injections before is that at baseline, none of them met ADA guidelines for glucose control. But at the end of the study, 55% of them did.
And so hopefully by now, I've convinced you that simulations are really useful in development and product design. But how do we build good simulations? Well, we build models. some are useful. So we try not to overmodel, not to undermodel. But we start with real world data.
So for example, at Tandem, we have data from 420,000 worldwide customers in our T:Connect system, we have 500,000 patient years of data. So we use all of that data from publications, from clinical trials to build models of physiology, of user behavior, of CGM, of different things related to diabetes management. And then we put these models together to run in silico clinical trials with our algorithm choices, with our design choices.
Here's another picture that's very similar to one you've seen in the previous talk. I didn't get go in much detail with some of the components, but these are some of the things we simulate. So we have the user's physiology, how their blood sugar responds to carbs, to insulin, to unmeasured disturbances that this system doesn't know about that generates blood glucose levels, which feed into a CGM model, which then generates CGM values that go into our classical algorithms, and into the pump to generate alarms and alerts, and to get displayed to the user. And the user in turn reacts to these glucose levels, reacts to the alarms and alerts, does their maintenance task based on this.
And then we also have models of when people eat, how much they eat, how many times a day, how good they are at carb counting, whether they ask for insulin when they do this, or whether they don't. So when we put all of this together, we're able to generate data similar to that that comes from a clinical trial.
And as I said, simulations and clinical trials are complementary. But there are certain questions that you could ask-- you could try to answer with both. But as much as possible, if you can use simulations to answer a question and answer it well, you will speed up medical device development by years and reduce your cost significantly.
So this is a back of the envelope calculation I did. It's very rough. And the reason that is is nobody really runs 2,700-subject real-world clinical trials. Pivotal studies in diabetes are usually a few hundred people. But these are typical simulations that we run when we look at our algorithms-- 2,700 subjects for 90 days.
So my back of the envelope calculation is that running a trial like this would take about three years and would cost about $24 million. But running a simulation in the cloud takes 6 minutes and costs $0.20 in Azure. So simulations can be 2,500 times faster and 120 million times cheaper than clinical trials.
Similarly, if you compare running simulations in parallel in the cloud compared to doing it on your laptop, you might think, OK, well, doing it on my laptop is so much better than clinical trials, and saving so much and so many dollars. Because of the multiple things you can simulate and because of how useful simulations are, it can take quite a bit of time. So simulations in a cloud can be 250 times faster and 50 times cheaper than if you run them locally.
And invention is always comes from necessity. So one example of when this really was sobering for us was at Dexcom when I was telling you about the FDA panel. Dexcom had about two months from when the panel was scheduled to put together this big body of evidence and go in front of the public and the FDA to show it. The simulations we wanted to run we estimated would take about 60 days on our laptops, which we were using at the time, so not a lot of risks there. Everything would have has to go perfectly, which is why, by working in the cloud, we were able to do the same simulations in 6 hours, and really do a lot more risk assessment, make sure we give them the the right arguments.
So even though it's not as contrasting of a difference as with clinical trials, it's also very, very useful. And it allows researchers to get their answers to their questions right away and creates a really fast feedback loop for innovation.
So in conclusion, simulations that are essential for automated insulin delivery device development, they can answer questions that clinical trials cannot. And by putting together the right package of evidence for safety that includes both clinical data for what it's good at and simulations for what they're best that, you can build the most effective package to go for an FDA submission to get devices approved. Thank you for listening. And are there any questions?